Beat by Beetles?
Four out of every five species of animals seams to have figured out how to avoid premature ageing of their joints, so why haven’t we?
Introduction
Osteoarthritis (OA), or degenerative joint disease is often attributed to the ageing-processes and thought to be a case of when the body simply outlive some of its less essential parts, like a car where the wheel-bearings starts to grind before the engine as a result of a manufacturing error, an accident or by exceeding its estimated milage. Because stiff and sore joints in the second half of life doesn’t have much of an effect on reproductive success, at least not today, it is also a common assumption that there have been insufficient selection-pressure for the preservation of joint-health up into old age, framing OA as an inevitable side-effect of a long life. Currently, genetics are though to account for around 30-65% of OA-risk and are together with old age, previous joint-injury, cumulative load and obesity the sociodemographic factors that have the strongest association with the disease, which, misleadingly, matches very well with the above car-analogy.1)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5832048/2)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3766936/3)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5525396/4)https://pubmed.ncbi.nlm.nih.gov/32342888/5)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4966641/
Pathologically, OA is defined as a progressive reduction in the (radiographic) joint-space between bones as a consequence of successive degeneration of interspaced cartilage (I will include degeneration of both synovial and fibrocartilaginous joints in the definition of OA in this article. I will explain why further on), and since cartilage is largely avascular, hence possessing very limited regenerative capabilities in case of repetitive mechanical stress and injury, the question as to why our joints wear our seem to be self-explanatory.6)https://www.sciencedirect.com/science/article/pii/S0169409X18303193 They wear out because we wear them out. However, this mechanical perspective does not align well with the anthropological data gathered from the last 8.000 years of human history, in fact they seem to be totally incompatible. Neither does it do justice to the roughly half a billion year long evolutionary history of the vertebrate joint-structure which is a much more clever and resilient design than commonly believed.
Prevalence Through Human History
There can be said to have ben three major shifts human life-conditions within a timespan of about 12.000 years, first the neolithic revolution with the transition form a period of more than two million years of hunting and gathering to an agricultural lifestyle, followed by the industrial revolution with the dawn of factories and machines, and then the transition to present modern society. The first two meant very big changes to human loading-patterns, as well as fluctuations in load intensity and frequency, but interestingly the rates of OA barely changed at all during this time, neither with the emergence of agriculture nor with factory-work and desk-jobs, and remained at a relatively constant level at around 6-8%, which could be considered somewhat acceptable for a two legged primate of our size, and for comparison the rates of OA documented in marine mammals such as dolphins are about 3%.7)https://onlinelibrary.wiley.com/doi/full/10.1111/joa.12620 But then in the last 50 years up until the early 21st century the rates of OA suddenly more than doubled to about 16%, and latest epidemiological data indicates that it is still increasing.8)https://www.pnas.org/content/early/2017/08/08/17038561149)https://www.oarsijournal.com/article/S1063-4584(20)30007-8/fulltext
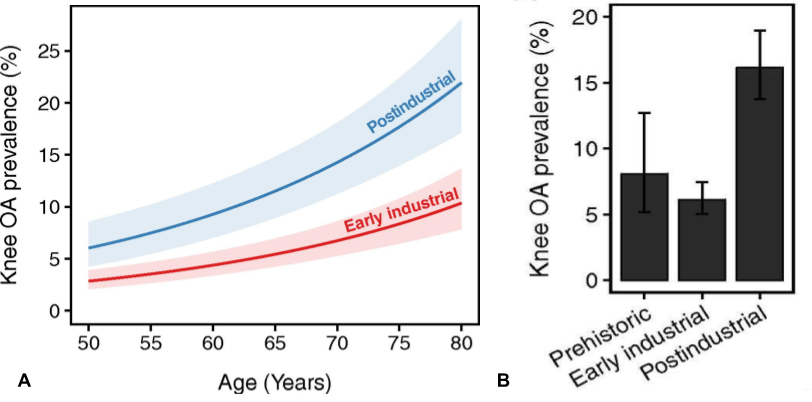
This recent surge in OA-rates is often assumed to be because we now grow heavier and live longer. However, a recent multivariate analysis have showed that increased BMI and longevity does not explain increased OA-rates associated with modern life, and the authors of this study instead speculate that it is rather the modern trend of physical inactivity that may account for current prevalence.10)https://www.pnas.org/content/early/2017/08/08/1703856114
The Physical Activity Paradox
Unfortunately, comparable data on physical activity levels isn’t available from before the 1950’s but from there on up until 2004 the most recent systematic review shows that levels of leisure-time physical activity increased while levels of occupational physical activity decreased during this period, also, studies that compare modern hunter-gatherers to industrial populations have found that they have a similar level of total energy expenditure (data controlled for body-size), which together suggests that the total difference in terms of accumulated activity might not be so large as commonly thought.11)https://pubmed.ncbi.nlm.nih.gov/19953831/12)https://onlinelibrary.wiley.com/doi/full/10.1111/obr.1278513)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3405064/
Contradictory Patterns
Epidemiologically those with higher levels of occupational physical activity actually seems to be at larger risk, while leisure-time physical activity (other than contact-sports) is generally considered to be protective against OA, however there is now data that shows that meeting the currently recommended physical activity guidelines is associated with a slight (but not statistically significant) increase in risk, and when looking at cross-sectional data that compare those with the highest versus lowest levels of leisure-time physical activity, radiographic OA actually seems to be more common in the former.14)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4309362/15)https://www.sciencedirect.com/science/article/pii/S1877065716300069 Prospectively, neither vigorous nor sedentary activity seems to be associated with increased risk of radiographic OA, and the difference between those that did low to moderate amounts of exercise versus those that either didn’t exercise or exercised a lot was also not significant over a 8 year period.16)https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2765373
A side-note is that OA is not the only form of degenerative disease, and if the root-cause of OA is wear and tear, or that it lies solely somewhere within the realm of mechanical load, we would then also have to assume that other degenerative diseases such as Alzheimer’s are because of too much/little use of the brain. In other words, just because a disease occur in mechanical tissues does not mean that there have to be a mechanical root-cause.
Confounding Effects of Obesity
Obesity (or rather metabolic disease) might be a variable that confound the relationship between physical activity and OA since it is associated with lower levels of physical activity and increased OA prevalence and severity with both high and low levels of activity.17)https://onlinelibrary.wiley.com/doi/full/10.1002/acr.21754
Osteoarthritis is Most Common in Non-Weight-Bearing Joints
The obese condition seems to have a dose-dependent relationship to OA of weight-bearing joints such as the knee, but it is also correlated with an increased risk of OA in non weight-bearing joints such as the hand, which is actually the most common location of radiographic OA.18)https://academic.oup.com/rheumatology/article/54/4/588/180051419)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2920533/20)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4966641/ This is very interesting since the hand is a structure that isn’t mechanically affected by the by obesity and was probably loaded equally or likely more vigorously and frequently in pre-historic and industrial times relative to the present (phone-thumbing and key-boarding included), making the hypothesis on physical inactivity very improbable.21)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2920533/
Osteoarthritis Occurs Only in Vertebrates
The origin of OA actually long predates the human time-line, so we probably wont find answers as to why the disease occurs in the first place by looking solely at human history, epidemiology and physiology. So we need to expand our perspective a bit.
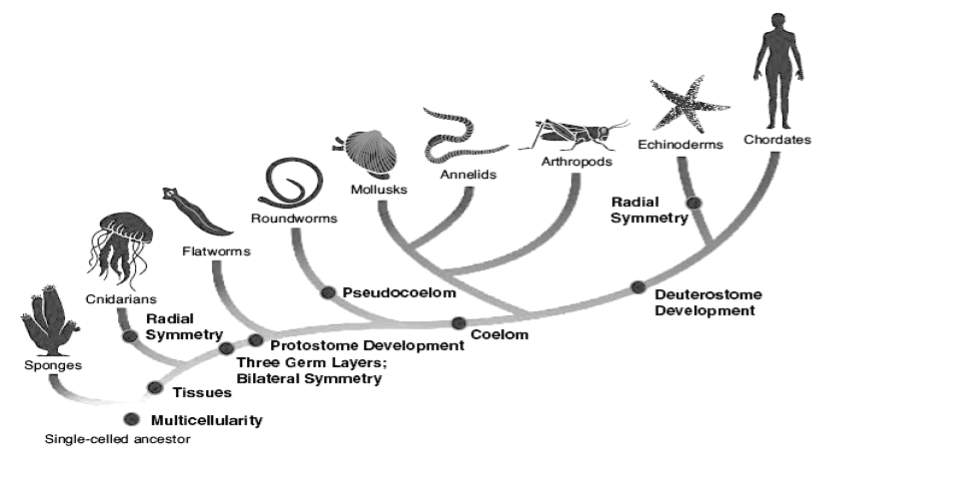
Humans are far from the only ones affected by osteoarthritis. In fact it has been shown to occur in most other classes of vertebrates with varying sizes and relationships to gravity including other mammals and species of fish, birds and reptiles, with the oldest case dating back about 120 million years.22)https://onlinelibrary.wiley.com/doi/full/10.1111/joa.1262023)https://www.sciencedaily.com/releases/2016/07/160714100149.htm24)https://www.sciencedirect.com/science/article/pii/S0195667111002084?via%3Dihub However, the prevalence of OA does not stretch beyond the vertebrate phylum because of two simple reasons. First, not all organisms need joints to move around, secondly, there are many forms of joints and only a small fraction of them have the necessary prerequisites for OA such as (avascular) cartilage that can degenerate and a joint-space than can narrow. This begs the question as to why did we evolved these features?
Comparison of Vertebrate and Arthropod Joint-Designs
Possession of joints is still the norm in the animal kingdom, but out of all species on the planet, more than 80% of them actually uses a joint-design that is quite literally the the exact opposite to that of vertebrates.25)https://www.britannica.com/animal/arthropod Grouped together these organisms are known as arthropods, and like the vertebrates they too inhabit both the air, sea and land, and can like us also live to become over a 100 years old.26)https://www.livescience.com/55392-do-lobsters-live-forever.html. Judging by the prevalence of the arthropod blue-print in organisms, their sheer numbers, and the range of eco-systems inhabited, biologically they must have gotten a few things right, and durable joint-physiology might be one.27)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3481597/
In contrast to the joints in vertebrates that are defined by opposing surfaces of bone interspaced with hyaline- or fibrocartilage that is largely cut-off from the normal vascular supply, the joints of arthropods neither have nor need joint surfaces or cartilage and are constantly flushed with nutrients by an open vascular system that runs straight through their hollow limbs which consist of a hard outer shell (known as exo-skeletons) with a few softer spots, or folds that allows for series of uni-planar articular rotations.28)https://pubmed.ncbi.nlm.nih.gov/31585504/ Cleverly their appendicular muscles, tendons and nerves also lies on the inside of this shell, which provides protection of these sensitive tissues from direct mechanical trauma. This design is basically like a vertebrate segmental joint-structure turned inside out and it makes the development of OA a theoretical impossibility, at least based on the current pathophysiological definition. Still to this date, there have been no documented accounts of anything approximating OA occurring in Arthropods. Absence of evidence is certainly not evidence of absence and arthropods probably aren’t immune to the effects of ageing on tissue-function, and there is a possibility that there might be a inside-out analogue to OA (why will become apparent further on in this section), but if this occurs it probably does not have as severe mechanical consequences as in vertebrates.
The exoskeletal design that are present in arthropod limb-segments has ben shown to possess excellent biomechanical properties, especially in relationship to its weight and they make most endo-skeletal morphologies that are present in the vertebrate phylum seem very clumsy and inefficient in comparison.29)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3481597/ Some variants of arthropod exoskeletons also have properties that makes near optimal use of elastic recoil which makes these organisms able to surpass the limitations of muscle-contraction and achieve impressive feats like the flea who can jump a distance that is 50 times its own body-length. This is because of the integration of specialised proteins into their exoskeletons, which have been shown to behave almost like perfect rubber and are unmatched both in biology and industry in terms of resilience and have a fatigue-limit of over 300 million cycles in-vitro.(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5082342/)) However, like in both biology and construction, there are trade-offs that come with every trait.
Biomechanics
Optimal skeletal design depends on mechanical demand, and in this regard the arthropod leg-structure is more optimised towards generating and resisting load in tension, and they are architecturally inferior to the cartilage-lined endoskeletal design present in vertebrates when it comes to the ability to withstand compression.30)https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0159262#abstract0 This is especially true at the joints, precisely because of the lack of joint-surfaces in arthropods.
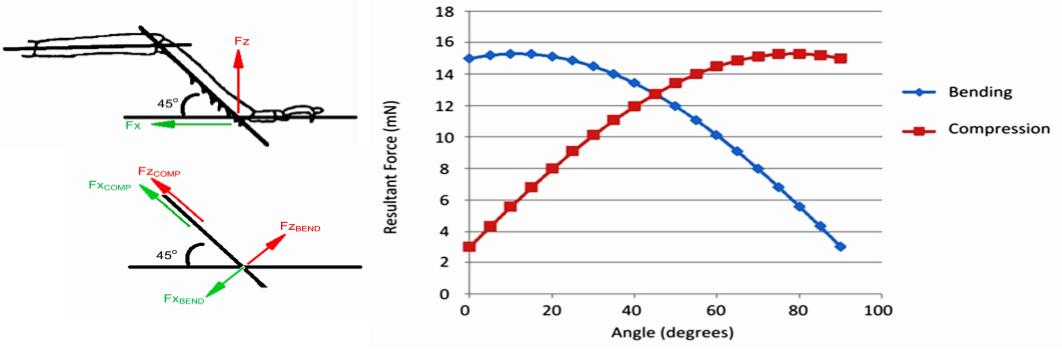
The ability to withstand higher compressive loads may be a major reason for the evolution of the vertebrate joint-morphology which enabled them to generate proportionally larger forces at their joints which in turn also allows them to grow larger in both in terms of size and mass. Flexibility is another advantage of vertebrate joint-designs, and in contrast to arthropod legs where each joint only has one linear axis of rotation, most vertebrate joints have the ability to execute multi-planar articular motion which enables them to perform more complex mechanical tasks. The capacity of a joint to execute multi-planar motion in theory also makes them less prone to severe traumatic injury since the joint can then more effectively absorb kinetic energy coming from varying directions. When comparing tolerance to stress, studies on mammalian bones has shown that they possess a safety factor of between 2-4 for static loads, but for repetitive load it is less than one. This means an overcapacity that is two to four times the levels of normal in-vivo exposure-rates in static loads and that the normal levels of longterm stress would actually cause gradual fatigue-failure of the tissue. Fortunately this is rescued by a continual process of self-repair. In contrast, arthropod legs have a safety-factor of 6-7 in normal longterm loading-patterns, which in the case of the locust can consist of constant running at a pace of eight strides per second for up to two hours going at a speed of over 3 km/h, accumulating over 57.000 successive load-cycles. However, the relative tolerance of arthropod legs to static or sudden loads (like a jumping motion in the locust) is substantially less, ranging from between 1.7 to 3, which is comparable some of the weakest values of structures in the field of mechanical engineering. This again points towards an advantage of the exoskeletal designs in terms of durability, but this is achieved by a compromise with robustness.31)https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0159262#pone-0159262-t00532)Comparative Biomechanics: Life’s Physical World – Second Edition By Steven Vogel
Thermodynamics and Hydraulics
Exoskeletons also serve non-biomechanical functions such as conservation and absorption of heat and reduced rate of water evaporation.33)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5438579/ However, these traits may complicate thermoregulation in response to internal and external temperature-fluctuations which is likely why most arthropods prefer cooler or more homeothermal conditions such as in shady and aquatic environments.34)https://www.britannica.com/animal/arthropod The limited ability to dissipate water and endogenously rendered heat may also be a reason for the apparent size-limit on the arthropod phylum since larger bodies generate more heat.
Metabolic Cost of Growth
Another trade-of with having an exo-skeleton is that it puts a limit on growth, or at least makes it more metabolically costly because it requires molting, while organisms with an endoskeleton can both up- and downsize more easily and dynamically since they carry their more expansive tissues, such as fat and muscle on the outside.
Reasons for Avascularity in Vertebrate Joints
The above reasons makes it clear why we don’t have a similar joint-physiology to arthropods, but what has not yet been made clear is why evolution in the over 500 million year history of vertebrates didn’t think about fitting cartilage with a proper vascular supply, which would up to this point, at least theoretically seem to negate some of the disadvantages in terms of durability of vertebrate joints.
Vascular Physiology Is Incompatible With Large Compressive Loads
The easy answer to this question is that blood-vessels can’t handle large compressive loads. For instance, it takes only about 2 Newtons, or 0,2 kg of pressure with a well placed thumb to occlude the brachial artery of the average person, and for arterioles and capillaries it’s even less. For comparison, pinching your thumb and index-finger to hold a 1 kg weight transmits around 5-12 kg of compressive load across each of the small joints in the hand and wrist, and when performing a strong grasp those forces can reach well above 100 kg.35)https://pubmed.ncbi.nlm.nih.gov/833171/ In simple walking the tibiofemoral portion of the knee-joint transmits forces that is 2-3 times the persons body-weight which increases by a factor of three during more vigorous activities such as running (which is about 100-250 kg and 300-750 kg respectively for a normal-sized human).36)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3324308/ Because large joint-loads are such a big part of our daily existence, it is therefore not possible for the nutritional needs of internal joint-tissues to be met by normal means.
Blood-Vessels Compromise Tissue Mechanical Properties
The presence of blood-vessels is also thought to compromise biomechanical integrity of cartilage because of the relatively large mechanical demand that is placed on this tissue per unit of mass and volume. As previously stated, blood-vessels are one of the tissues in the body that is the least suited to resist compressive load, and their presence, especially in articular cartilage would therefore blunt the impressive effectiveness of this tissue. Because of this, healthy chondrocytes continually synthesise and release molecules that directly inhibit angiogenesis, meaning that joints actually strives to remain avascular, and the resident cells have adopted a phenotype that can function in very low oxygen-concentrations of between 1 and 5%.37)https://pubmed.ncbi.nlm.nih.gov/11193203/
Cartilage Nutrition
Because of its avascular nature, cartilage was previously long considered to be a “dead tissue” unable to receive nutrients from the blood and was it was therefore though that it would inevitably and successively wear down with use. Fortunately, cartilage does have a way to interact with the vascular system through a very clever mechanism, which is unfortunately also the achilles-heel in our joint-physiology.
Generation of Synovial Fluid Through the Capsular Vascular Supply
Even though cartilage is largely avascular, the surrounding tissues are very vascular, especially the capsules of our synovial joints which acts like an intermediary in articular cartilage nutrient-supply by functioning as a filter that concentrates blood-plasma into synovial fluid which then disseminates into the joint-space to provide cartilage-cells with necessary nutrients to uphold tissue-homeostasis, mediate communication-signals through cytokines as well as also serving as a lubricant.38)https://www.sciencedirect.com/science/article/pii/S8756328212000683?via%3Dihub
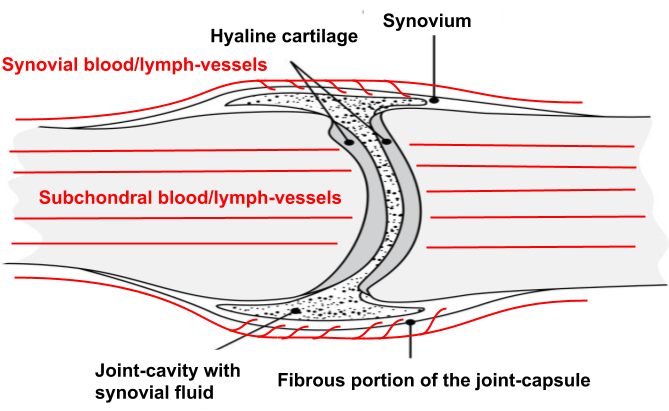
The lubricating function is aided by macromolecules such as hyaluronic acid which are synthesised and secreted into the joint-space by specialised cells on the inside of the synovial membrane. Hyaluronic acid is found in most other places of the body where tissue-surfaces move along each other, and its main function is to create a layer of physical separation between tissues and to prevent the formation of adhesions, which is essential for normal joint-, muscle- and tendon-function.39)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3967437/
Plasma-Filtrate From Subchondral Vessels
There is a second route that carries nutrients to articular cartilage which comes from arteries running through the bone that then form capillary beds beneath the cartilage surface, generating a similar plasma-filtrate but without the before mentioned macromolecules. Studies have looked at the relative contribution of the synovial and subchondral vascular routes and have concluded that the synovial portion seems to be the main source of nutrients. By experimentally blocking either route in an animal model over an extended period of time they showed that cartilage-loss occurred in both scenarios, but that it was much more severe when the only source of blood came from the subchondral arteries.40)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3717153/ This is probably because of the deepest layer of mature articular cartilage being calcified and hence not as permeable as the synovial membrane.41)https://www.sciencedirect.com/science/article/pii/S1063458407003160
Diffusion From a Direct Vascular Supply
Some fibrocartilaginous structures such as the intervertebral disc, menisci and the pubic symphysis have a direct vascular supply in their peripheral parts.42)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1571274/ This is because they mostly resist hydrostatic forces which loads them in tension rather than compression when the whole structure is exposed to axial load, which allows for the presence of small blood-vessels that track centrally until compressive forces become to large.43)https://link.springer.com/article/10.1007%2FBF02328563 From the capillary beds, fluid-filtrate is generated similarly as in the two previous routes, but to reach into the avascular portion of the tissue all three need assistance of another variable namely mechanical load.
The Synergistic Effect of Mechanical Load
Nutrients can reach into the deeper portions of avascular tissues through two mechanisms, by diffusion and convection. Diffusion is when nutrients move within a fluid medium according to a concentration gradient, whereas convection is when nutrients move with the flow of fluids. Both of these modes of transport are affected by loading patterns, and prolonged static loads have been shown to inhibit nutrition because it leads to a decreased water-fraction in the tissue, whereas dynamic load has been shown to enhance nutrient exchange by inducing convection.44)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2748424/45)https://www.sciencedirect.com/science/article/pii/S1063458417311652 As with tissues that have a direct vascular supply, cartilage is also dependent in a continual turnover of nutrients, which means there has to be a way in which old fluid-filtrates can leave the joint and re-enter the circulation. This takes place mainly through lymphatic vessels in the synovial tissue or by direct venous return in the subchondral and peripheral blood-vessels. These return-flows probably depend a lot on mechanical load and the state of the capsule, subchondral bone and peripheral tissue-portions respectively to avoid congestion.46)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1005608/?page=147)https://www.sciencedirect.com/science/article/pii/S1063458412000362
The Pathomechanism of Osteoarthritis
Histopathologically, OA is characterised by local inflammatory change in the synovial- and subchondral tissues as well as in the synovial fluid.48)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6856926/ Considering the previously outlined mechanisms for cartilage nutrient-supply where the peripheral joint-tissues acts as a bottle-neck, inflammatory activity in this area might pose a very big problem.
Effects of Peripheral Inflammation of a Joint
Swelling is one of the hallmarks of inflammation and might severely complicate cartilage nutrient-exchange because it increases the diffusion-distance from the vascular supply to the joint-cavity and the internal structures of the joint.
For perspective, in synovial joint-membranes the arteries end approximately at a distance of 35 μm from the joint-cavity just below the 1-2 layers of cells that constitutes the inner lining, and oxygen which is essential for the survival of all cells in the body have a maximal diffusion-distance of only around 150 μm. Studies has also shown that a histological characteristic of OA is hyperplasia of synovial cells (which is probably a secondary consequence of hypoxia) and fibrotic thickening of the capsule, both which are down-stream effects of inflammation and can potentially further increase diffusion-distance and complicate convection.49)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3372675/50) … Continue reading51)https://www.jimmunol.org/content/184/2/1049
Inflammation of peripheral joint tissues thus threatens to block the supply of oxygen as well as potentially other essential molecules such as glucose for the whole inner portion of the joint, which is probably a major reason why it has proven to be strongly associated with OA.52)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5500215/
A Cause, Not a Consequence
Inflammation of peripheral joint-tissues was previously thought to be a consequence of OA rather than a cause, but a recent study showed the opposite to be true, that peri-articular inflammation actually preceded radiographic change in OA. This study examined data from The Osteoarthritis Initiative and compared people that showed no radiographic signs of OA at baseline who later went on to develop OA over a 4-year period with a control-group who did not show any radiographic signs of OA during this time. By using data from annual radiographs and MRI-scans they calculated the odds-ratio for developing OA when synovitis was detected on baseline-MRI to be 1.56, and when it was found one year prior to diagnosis and at the time of onset it was shown to be 3.23 and 4.70 respectively.53)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4916836/
Similar Effect on Synovial and Non-Synovial Cartilage
Inflammatory stimuli probably has a similar effect on both synovial and fibrocartilage structures since the common denominator in all nutrient supply-routes to cartilage is diffusion/convection from vascular sources in the peripheral parts towards an avascular center. In support of this, like in synovial joints degenerative changes in fibrocartilaginous structures such as the intervertebral disc seems to progress with an inside-out pattern, which is also true for other tissues with a similar mode of interaction with the vascular system such as tendons.54)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3527450/55)https://bjsm.bmj.com/content/50/19/1187
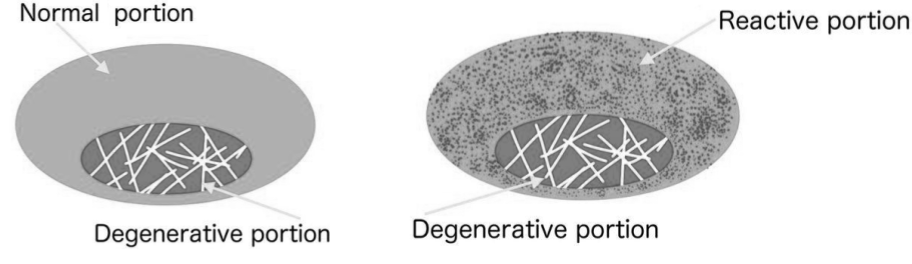
A study has also shown the presence of venous stasis and reduced arterial penetrations in the subchondral bone of osteoarthritic synovial joints, which suggests that the subchondral vascular route, which is a common vascular pathway for both synovial and fibrocartilaginous joints can be compromised in a similar way as the synovial membrane.57)https://pubmed.ncbi.nlm.nih.gov/29723635/
This argues that the pathophysiology of joint-degeneration is the same in synovial joints such as the knee, and non-synovial joints such as the intervertebral joints of the spine. Inflammation at the tissue-vascular interface is probably also part of the pathophysiological pathway in tendinopathy and perhaps also in degenerative diseases outside of the locomotor-system.
Chondrocyte Starvation
The end-effect of a compromised nutrient exchange is starvation of the resident cell-populations, and in line with this chondrocytes taken from animal models of OA have been shown to be critically low on intracellular levels of energy.58)https://onlinelibrary.wiley.com/doi/pdf/10.1002/art.20149 Starvation of chondrocytes has a deleterious effect on cartilage mainly through two mechanisms. First as a direct consequence of energy-deprivation which makes them less efficient at tissue maintenance-functions, and secondly because starvation induces a state of chondrocyte autophagy which causes them to actively consume cartilage extracellular matrix-proteins for survival. In support of this, there is evidence of endogenously mediated cartilage-destruction in response to inflammatory stimuli from both in vivo and in vitro studies.59)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC437042/60)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1887353/pdf/amjpathol00065-0258.pdf61)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC387009/ Vascular congestion isn’t the only way in which inflammation cause cartilage nutrient-deprivation and destruction, there might also be a role for infiltration immune-cells.62)https://www.ncbi.nlm.nih.gov/pubmed/2750224563)https://www.ncbi.nlm.nih.gov/pubmed/31084709
The Role of Infiltrating Immune-Cells
Besides edema, another characteristic feature of inflamed tissues that affects parenchymal nutrient-availability is accumulation of immune-cells, which in the case of OA has been observed both in the synovial membrane and in the subchondral bone. These cells show an increased inflammatory activity relative to their resting state and may exacerbate the effect of vascular congestion on cartilage tissue by stealing nutrients as well as consuming local tissue-proteins as means to drive necessary immunologic functions related to the inflammatory stimuli.64)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7013211/ This is part of a mechanism I have discussed previously in the model-chapter referred to there as “nutrient partitioning” where parenchymal cells are shown to down-regulate nutrient import-mechanisms to increase availability for infiltrating immune-cells. The mechanism of insulin resistance and hyperglycaemia in type-2 diabetes mellitus might work in a similar manner, to increase the proportion of energy directed to the immune-system.
Inflammatory and Degenerative Pathology, a False Dichotomy.
The mechanism of peripheral inflammation causing central degeneration might explain the confusion in the scientific community as to the nature of OA which was long considered to be a non-inflammatory, degenerative disease.65)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3638313/ As I’ve explained in the model-chapter, degeneration is an inevitable side-effect of ongoing inflammation but this connection is is less obvious in joints because of their relationship to the vascular system. Histologically the changes in the avascular parts mostly take on the characteristics of oxygen- and nutrient-starvation, while inflammatory change is mainly observed in the peripheral, capsular structures, but it is also a great deal of overlap between the two.
Since the most striking histopathological change is observed in the cartilage, it is not so hard to see why the inflammatory component of the disease was missed and refuted in earlier times. Now however there is very little controversy around OA being an inflammatory disease with degenerative features.
Evidence of a Systemic Root-Cause
Since joint-inflammation seems to precede joint-degeneration it begs the question as to what causes the inflammation. A recent study that looked at biomarkers of low-grade inflammation such as highly sensitive C-reactive protein (hsCRP) in synovial fluid and synovial tissue of osteoarthritic joints found a correlation with increased serum-levels of the same biomarker and also other inflammatory mediators such as interleukin-17 which implies that there might be a systemic trigger for the local inflammatory response observed in OA.66)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6856926/
C-reative protein (CRP) is not just a biomarker for inflammation, which is presently its most common domain of use in diagnostic medicine. Its an acute-phase protein that is directly involved in the anti-infectious response and has been shown to rescue test-animals from lethal bacteremia.67)https://www.frontiersin.org/articles/10.3389/fimmu.2019.00166/full In line with this, studies have shown that traces of bacteria are elevated in serum and also in joint-tissues and synovial fluid of OA-patients, correlating with both objective and subjective markers of disease-severeity.68)https://www.oarsijournal.com/article/S1063-4584(16)30086-3/fulltext
Because of the special relationship between joints and the vascular system, the body has a very hard time to clear out an infection that has reached a joints inner portions, and pathogens that manages to gain access to this part therefore have the potential to grow a high level of virulence by having virtually unrestricted access to nutrients stored in cartilage-tissue and synovial fluid. This might be a reason why true septic arthritis usually cause a massive systemic inflammatory response and also why it has a fatal outcome in about 66% and 20% in untreated and treated cases respectively.69)https://pubmed.ncbi.nlm.nih.gov/21916390/ The virtually non-existent regenerative capacity of articular cartilage, and the essential functions mediated by effective joint-movement also makes the consequences of a pathogenic infection very serious even if the host-organisms survives the initial insult, which further ads to the gravity of the situation.
An interesting observation related to septic arthritis is that the most commonly affected joints are the knees (46%) followed by the hips (31%), which are also the second and third most common location of symptomatic OA.70)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4228814/71)https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4384650/ These are also the joints in the body that are subjected to the greatest levels of peak biomechanical stress (after the ankle joint) with normal daily activity which could mean that areas of high tissue-turnover or nutrient-needs are of particular interest to pathogens.72)https://link.springer.com/chapter/10.1007/978-1-4939-0745-8_8 Studies have also shown that a majority of patients that present with septic arthritis also have pre-existing inflammatory joint-disease, which could mean that low-grade joint-inflammation is just an early stage of a more threatening infection and that these diseases are actually more like prodromal symptoms of potential septic arthritis.73)https://ard.bmj.com/content/56/8/470 Why the joints of the hand and ankle are not as commonly affected by septic arthritis despite being the most common location of OA and the joints subjected to the larges level of peak biomechanical stress (in walking and running) respectively might be because of the difference in hemodynamics between small and large joints, resulting in a smaller area of microbial exposure and a lower level of total septic load in the former. However, this is purely speculative.
What this all indicates is that the periarticular inflammation that underlies OA might be an attempt by the body to block and clear out low-virulent pathogens that are trying to access the joint-cavity, which must be considered a high-priority task for above reasons, even though it results in a temporary (or persistent) compromise with cartilage nutrition leading to decreased durability.
Summary
As the needs to bear greater axial compressive loads by our first vertebrate ancestors rose with increasing body-size and mass over time, so did the need to develop avascular joint-structures. The anatomical and physiological blueprint of both the synovial and non-synovial vertebrate joint have since been molded over the course of over 500 million years to suit our biomechanical needs, and to last reasonably well, at least as long as we do. The prerequisite to this is that we stay out of trouble, meaning the avoidance of infection and injury, which otherwise compromise both the quality and the longevity of the tissue. Sub-clinical infection that causes low-grade inflammation is probably the major cause of osteoarthritis, which is in turn caused by alterations to dietary patterns, sleep-habits and others life-style-related variables as well as environmental factors associated with measures of general health, which are the true root-causes of disease.
For a more detailed explanation of how inflammation relates to disease and what causes it see the sections on Pathomechanism and Etiology under the Model-chapter.
References
